
Physikalische Chemie - Direktor: Prof. Dr. Martin Wolf
Special Seminar
Host: T. Kumagai
Monday, July 24, 2017, 11:00 am
All are invited to meet around 10:40 am for a chat with coffee & cookies.
PC Seminar Room, G 2.06, Faradayweg 4
Dr. Leo Gross
Zurich Research Laboratory, IBM, Zurich
Investigating Radicals and Charge Manipulation with AFM
The combination of high-resolution AFM with atomic manipulation offers the possibility to custom-design individual molecules by making and breaking bonds with the tip of the microscope and directly characterizing the products on the atomic scale.
We recently generated and studied reaction intermediates and investigated chemical reactions trigged by atomic manipulation. We formed radicals by dissociation and reversibly triggered ring-opening and -closing reactions via atomic manipulation. By STM and AFM we address long-standing debates about the structure, aromaticity and the open-/closed shell character of radicals, antiaromatics and non-Kekulé molecules.
On multilayer insulating films we investigated single-electron attachment and detachment and charge transfer between individual molecules
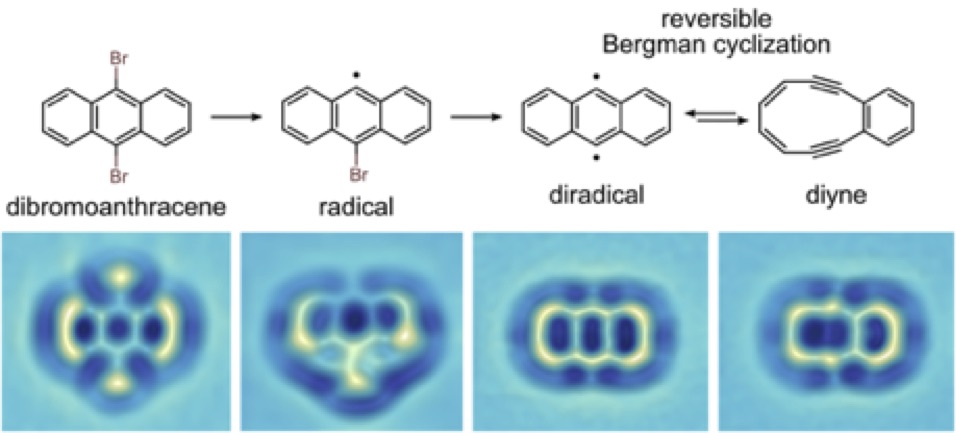
Figure: Reversible Bergman cyclization by atom manipulation: Chemical reaction scheme (top) observed for an individual molecule using an AFM with CO tip functionalization (bottom). All reaction steps are induced by bias voltage pulses with the AFM tip. The initial product dibromoanthracene on bilayer NaCl on Cu(111) is first debrominated, then a Bergman reaction can be reversibly triggered at voltage pulses above 1.6 V. The molecule can be switched between diradical, which features a spin triplet ground state, and diyne, which features a spin singlet ground state. (Nat. Chem. 8, 220, 2016).
We recently generated and studied reaction intermediates and investigated chemical reactions trigged by atomic manipulation. We formed radicals by dissociation and reversibly triggered ring-opening and -closing reactions via atomic manipulation. By STM and AFM we address long-standing debates about the structure, aromaticity and the open-/closed shell character of radicals, antiaromatics and non-Kekulé molecules.
On multilayer insulating films we investigated single-electron attachment and detachment and charge transfer between individual molecules

Figure: Reversible Bergman cyclization by atom manipulation: Chemical reaction scheme (top) observed for an individual molecule using an AFM with CO tip functionalization (bottom). All reaction steps are induced by bias voltage pulses with the AFM tip. The initial product dibromoanthracene on bilayer NaCl on Cu(111) is first debrominated, then a Bergman reaction can be reversibly triggered at voltage pulses above 1.6 V. The molecule can be switched between diradical, which features a spin triplet ground state, and diyne, which features a spin singlet ground state. (Nat. Chem. 8, 220, 2016).