Time- and angle-resolved photoemission spectroscopy
Angle-resolved photoemission spectroscopy (ARPES) provides the most direct access to the electronic structure of solids. The measurement of the energy and the emission angle of electrons emitted by light with photon energy above the work function, typically extreme ultraviolet (XUV) light, allows for the determination of the material’s electronic structure.
Employing ultrashort pulses of XUV light in combination with a second ultrashort visible laser pulse adds temporal resolution to this technique: trARPES provides movies of a material’s electronic structure with a frame rate of a few tens of femtoseconds. In cooperation with Martin Wolf and the research group Dynamics of Correlated Materials headed by Laurenz Rettig, we recently advanced this technique by developing a novel XUV laser providing ultrashort pulses of ~22 eV photon energy with 0.5 MHz repetition rate.
In a trARPES experiment, a small fraction of the electrons of the material is excited by
Ultrafast electron dynamics in 2D materials
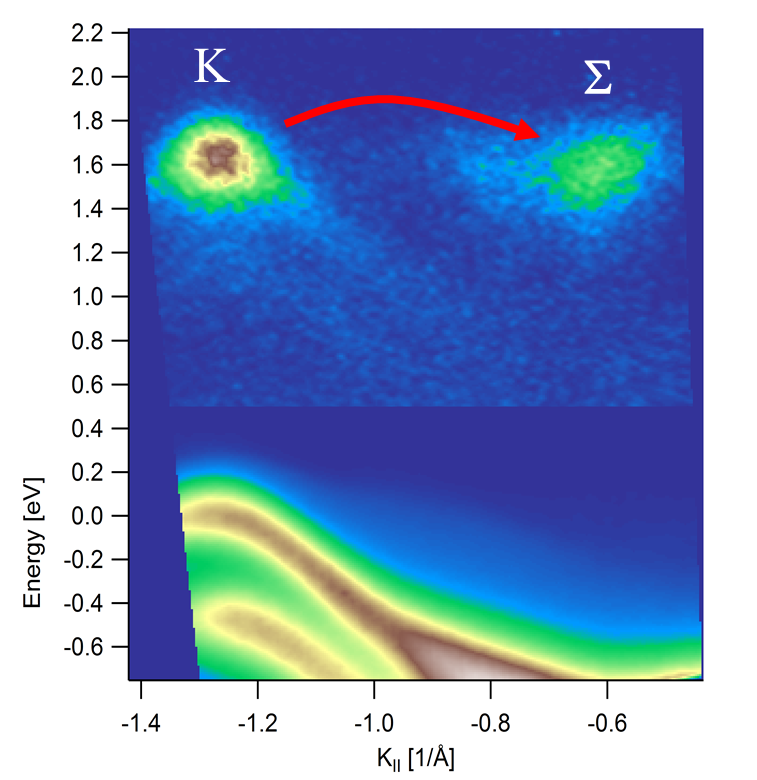
Fig. 1: Screenshot of a trARPES movie. Electronic Structure of WSe2 shortly after excitation of electrons in the K valley of the band structure. The bottom part of the image shows the occupied valence bands. The signal above 0.5 eV energy is rescaled by more than a factor 100 and shows the electrons excited by a near-infrared laser pulse. The red arrow indicates intervalley scattering of electrons from the K to the Sigma valley in the Brillouin zone, which occurs in less than 100 fs.
XUV trARPES experimental setup
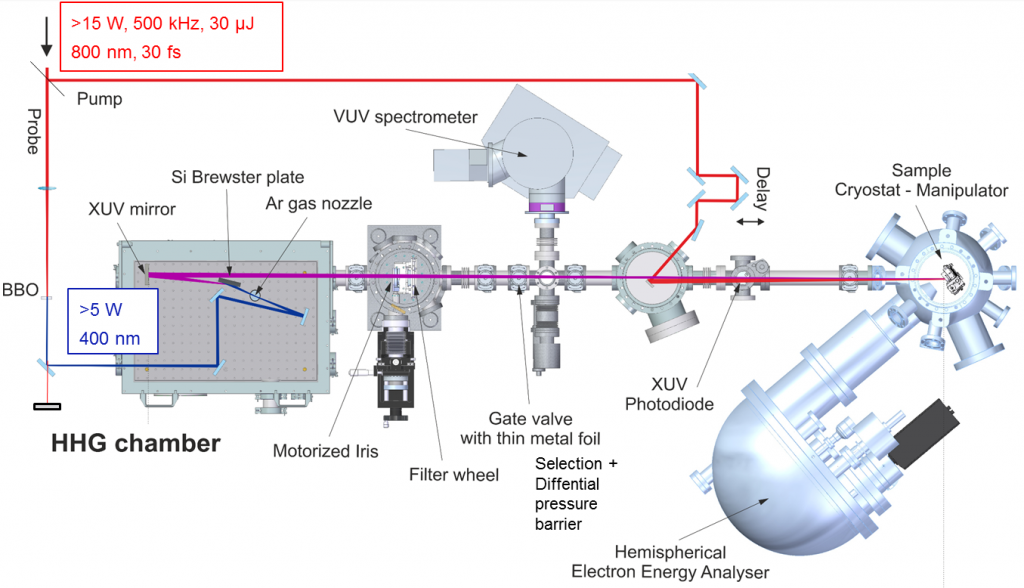
Until recently, there have been two branches in the implementation of trARPES, one based on high repetition rate laser systems providing high counting statistics but limited probe photon energies, which limits the accessible range in the Brillouin zone, and another based on probing with extreme ultraviolet (XUV) light generated by high harmonic generation employing high power laser systems of limited repetition rate.
In order to bridge this technology gap, we developed a 0.5 MHz XUV laser source based on high-harmonic generation (HHG) driven with the output of a femtosecond parametric laser amplifier. Its output is frequency-doubled to yield 10 µJ pulses centered at 400 nm wavelength. These pulse are focused into a jet of Ar atoms where frequency up-conversion occurs through HHG. The 7th harmonic of the 400 nm (photon energy: 3.1 eV) pulses is filtered by a combination of XUV mirror and transmission filter and focused on the sample to trigger photoemission. The XUV laser pulses (photon energy: ~21.7 eV) have a pulse duration of approximately 20 fs and a bandwidth of 100 meV.
To learn more about the setup, please read our relevant publications. or check additional details here.
In/Si nanowires
Watching the motions of atoms in the course of a chemical reaction is generally thought of as the Holy Grail for understanding chemical transformations or phase transitions in solids. While recordings of such “molecular movies” have been achieved in recent years, the atomic motion does not reveal the whole story of why specific bonds break and others form. This is dictated by the arrangement of the electrons as the atoms move along gradients on an energy landscape defined by the electrons. It is therefore necessary to observe the dynamics of the electronic structure, which means to record an “electron movie”, to obtain a complete picture of the mechanisms driving chemical reactions.
An experimental team at the Fritz-Haber-Institut in Berlin and computational scientists at the University of Paderborn now filmed the electrons during a light-induced reaction. They investigated a single layer of indium atoms on top of a silicon crystal. At low temperatures, the indium atoms form an insulating layer with the atoms arranged as hexagons. At room temperature, however, the indium atoms rearrange and form conducting atomic wires. This phase transition can not only be induced by changing the temperature but also by exciting the cold material with a very short flash of light. This light pulse puts energy in the electrons of the material faster than the atoms can move. Due to the extra energy, the electrons reorganize and change the energy landscape for the atoms: the atoms immediately start to move. In turn, the swift electrons react to the change in the atomic structure. This dynamic interplay between electrons and atoms has been recorded with time- and angle-resolved photoemission spectroscopy: a second ultrashort laser pulse is used to emit few of the electrons at different times after the phase transition was initiated by the first laser pulses. By repeating this process billions of time, a movie of the electronic structure during the phase transition of the indium nanowires was obtained. This information, combined with simulations of the electronic structure dynamics, made it possible to translate the electronic structure dynamics into a movie of the atomic energy landscape. This detailed reconstruction of the reaction pathway reveals not only the motion of atoms but also the formation and breaking of chemical bonds during the phase transition.
The approach demonstrated by Nicholson et al. is generally applicable to physical processes like structural phase transitions in solids as well as to chemical reactions, for instance of molecules. The theoretical framework for describing the electronic structure, however, differ significantly between these cases: while electrons in a crystal are described as bands in momentum space, electrons in molecules are depicted as bonds in real space. The work by Nicholson et al. provides a bridge between the languages of physics and chemistry for describing photo-induced reactions. Understanding how the transient electronic structure results in bond dynamics may in future allow the tailoring of chemical reactions and phase transitions via engineered light pulses.
“Beyond the molecular movie: Dynamics of bands and bonds during a photoinduced phase transition”
Science, Vol. 362, Issue 6416, pp. 821-825
DOI: 10.1126/science.aar4183
http://science.sciencemag.org/content/362/6416/821
BigMax: Handling large Multidimensional Photoemission Spectroscopy Dataset
