Towards the investigation of heterogeneous reactions on static liquid-vapor interfaces
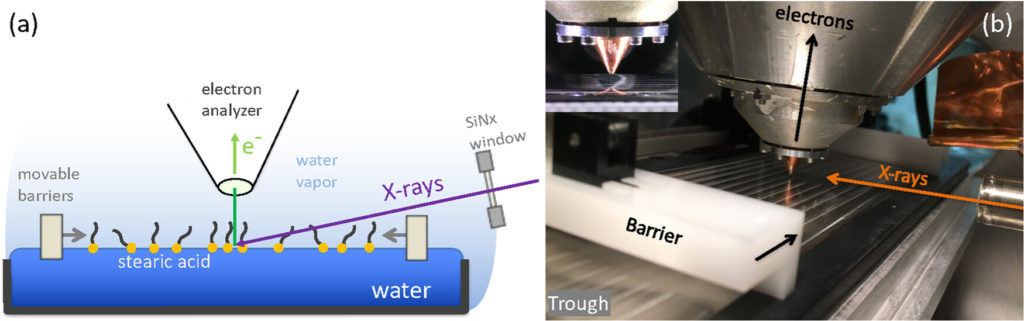
The relevant time scales for reactions between gas phases species (e.g., trace gasses in the atmosphere such as O3 or CO2 or NO2) and aqueous solutions are often minutes to hours for a full equilibration of the reaction products at the solution-vapor interface and in the bulk of the solution. We are developing strategies for the investigation of these reactions, using static aqueous solution samples (in contrast to fast flowing liquid jets and droplet trains) in contact with the gas phase. A proof of principle measurement showed the feasibility of combining a Langmuir-Pockels trough with ambient pressure XPS to investigate the compression state of stearic acid surfactant layers on water. (Hoek et al., J. Phys. Chem. B 128, 3755 (2024))
Increased depth resolution in XPS investigations of liquid-vapor interfaces
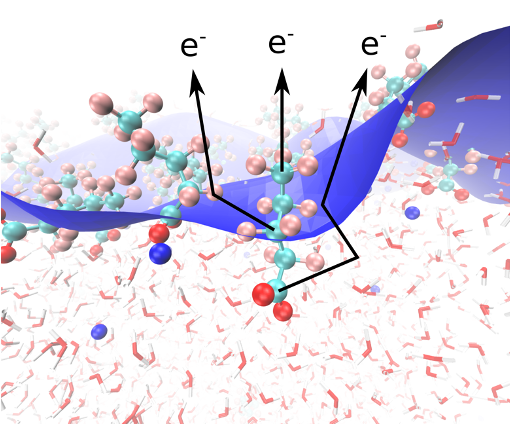
The depth resolution in photoelectron spectroscopy is conventionally governed by the inelastic mean free path of electrons in matter, which depends on the kinetic energy of the electrons and is in the case of water at least more than 15 Å. The formation of double layers at the liquid-vapor interface of aqueous solutions and the solvation of the hydrophilic headgroup of surfactants often happens on shorter depth scales, which poses problems for a detailed understanding of the depth profile based on XPS measurements. This problem can be overcome by measuring the degree of elastic scattering of electrons, which is sensitive to depth differences as low as 1 Å under favorable circumstances. This approach is based on the measurement of photoelectron angular distributions, where XPS spectra are measured as a function of the angle between the electric field vector of the incident X-rays and the electron detection direction. (Dupuy et al., Phys. Rev. Lett. 130, 156901 (2023); Acc. Chem. Res. 56, 215-223 (2023); Phys. Chem. Chem. Phys. 24, 4796-4808 (2022))