Aqueous Solution-Vapor Interfaces: Modeling the Ocean
The interaction between the oceans, aqueous aerosols, and the air is crucial for many physical and chemical processes, such as the absorption of CO2 by the oceans. The transport and reactions at the ocean-air boundary are largely influenced by the chemical makeup of the interface, specifically the first few nanometers into the ocean. This interface mainly consists of water molecules, dissolved ions, and amphiphilic surfactants, which are commonly found in nature. By using a combination of surface tension measurements and liquid jet X-ray photoelectron spectroscopy, we have studied model seawater solutions both with and without model surfactants. Our research offers a detailed view of how the presence of charged surfactants at the interface affects the concentration of ions, either enhancing or reducing it. Additionally, the concentration of surfactants at the interface is directly linked to the ionic strength of the solution, known as the “salting out” effect. The interactions between ions and surfactants can significantly alter their concentrations at aqueous solution-air interfaces, impacting heterogeneous reactions and the uptake and release of gases at ocean-air boundaries.
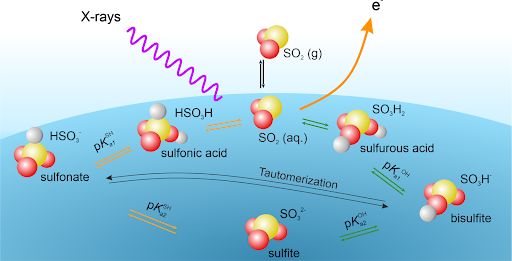
Atmospheric Interfaces: The Sulfur Cycle
Within the sulfur cycle in the atmosphere, S(IV) species are in a complex acid-base equilibrium, which also includes tautomers and short lived species in the aqueous phase. Sulfite can potentially be oxidized in a heterogeneous reaction to sulfate and nitrous acid, which are key molecules in the atmosphere (nucleation and radical sources). We investigate these equilibria at the interface and compare them to the ones in the bulk. We also aim to monitor and analyze in detail the formation of the short-lived nitrous acid (HONO) from sulfite and NO2 gas.